Biomaterials Growth
One important problem in the biomineralization processes is the interaction between biological macromolecules and mineral surfaces due to the modifications introduced by the biomolecules in the mineral growth thermodynamics. These modifications are able to change the preferred growth orientations modifying the crystalline habit. In the present study we investigate the relationship between calcite ionic crystal and the polyanionic oligosaccharide dermatan sulfate (DS), which is found in avian eggshells [1]. With the help of template substrates of DS biomolecules we get insight about how DS coordinates on calcite crystal surfaces. The topography of the template is obtained in air with tapping mode atomic force microscopy (TM-AFM) and the surface charge density of the substrate in an aqueous solution using the microsphere cantilever technique in an AFM. Then, the DS substrate surface is activated with CaCl2 and the crystal growth facilitated by adding a supersaturated salt solution. The most favourable sites for nucleation are those able to adsorbe Ca2+ ions [2]. These sites are found in the anionic groups (sulphate and carboxilate) of DS chains. Thus, the crystal nucleus orientations are closely related to the electrostatic charge density on the substrate. Crystals of macroscopic dimensions are observed by visible light optic microscopy and SEM analysis allows us to measure and recognize the crystal angles and their orientations.
Experimental Procedures
- Substrate preparation
Freshly cleaved mica siloxane-silanol surface following the natural cleavage plane (001)

An (APTMS) 3-aminopropyltrimethoxysilane self-assembled monolayer is created on the freshly cleaved mica by silanization technique.
![]()
These molecules binds covalently with silane group to mica surface, and leave their amino groups to the upper end [3]. We measure topographically this monolayer in samples that were made at different concentrations; a height near to 1 nm was obtained and is show in the next air TM-AFM height image.
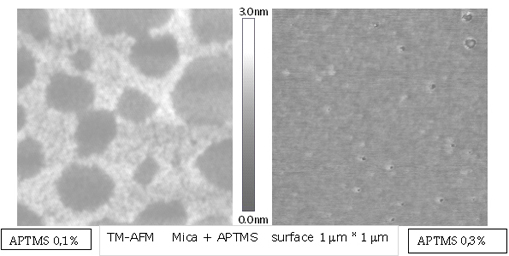
Over the homogeneously covered APTMS monolayer we add the DS biopolymer obtaining newly an homogeneously covered surface.
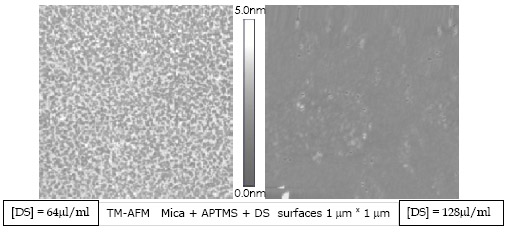
X-ray photoelectron spectroscopy (XPS) was used to verify that the substrate was formed in the expected way.
To measure changes in the surface mean charge density of the mica substrate (non covered and covered with APTMS or DS) we faced an amorphous silicon dioxide (glass) microsphere [4] covered with a similar molecule against the substrate. Then depending on pH, the ionizable groups of the molecules of interest have different charge states producing a repulsive electrostatic force, in opposition to the van der Waals force, which is measured by an AFM in force spectroscopy experiments [5]. This measurement is done in each stage during the substrate formation.
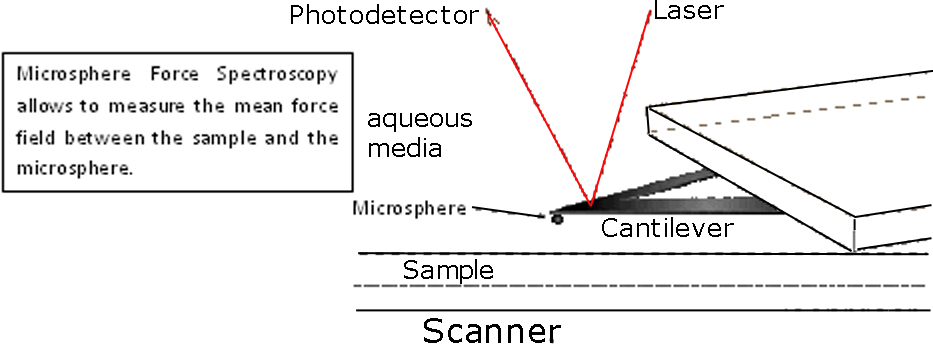
In our case we can fit the going to contact force curves with the following model:
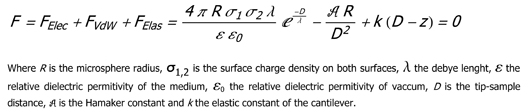
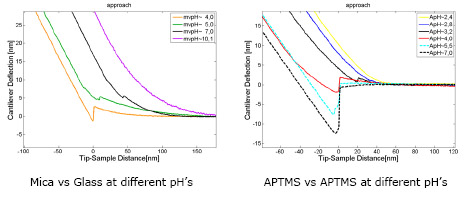
The amino groups remain positively charged until pH<6, while the DS molecules are charged negatively since pH>4
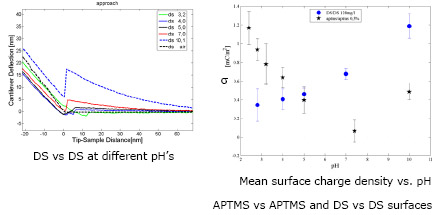
We can see that at pH=5 APTMS and DS have enough charge to be attracted electrostatically during the substrate forming process.
Once the DS substrate is prepared it is activated with a solution of CaCl2 [6] that growth as soon as a supersaturated solution of salts is added [7]. The crystalline phases and orientations are analized through AFM, SEM and BSE
| Early orientational stage after calcium carbonate nucleation process on DS substrate |  |
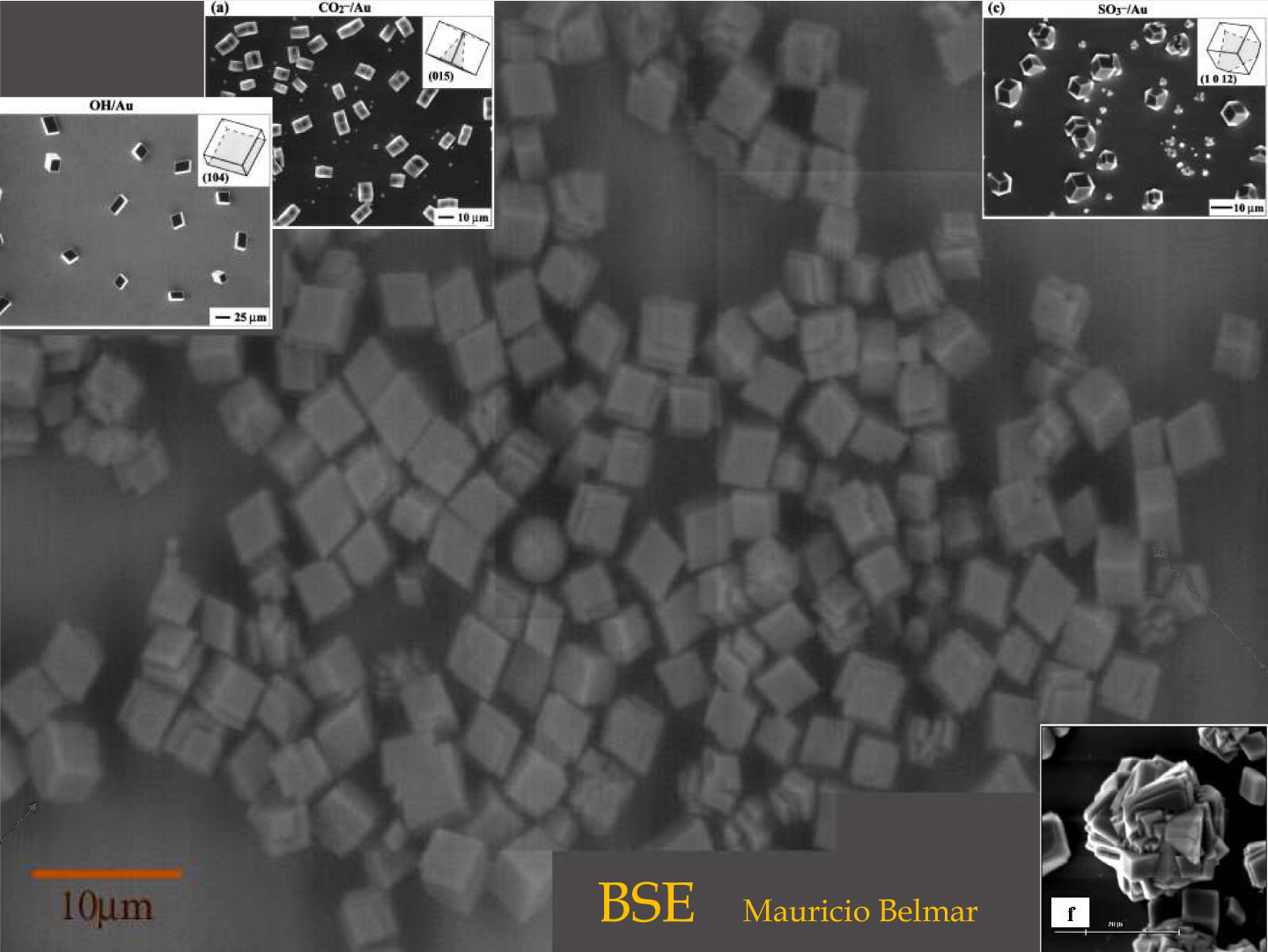
Corner images are from the cited articles [1] and [6]
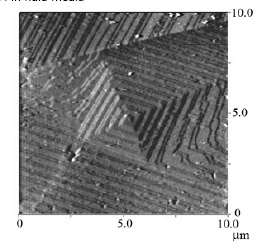
2.- in-situ addition of DS to the crystalline growth of a screw dislocation on plane (104)
We study the role of biological molecules on the shape, interfacial properties and structures of calcium carbonate. We have developed simple methods to obtain a well defined Calcite cleaved planes. The figure below shows in situ screw dislocation growth of Calcite on the (104) plane, obtained with contact mode AFM in fluid media
References:
[1] J.L.Arias et al J. Mater. Chem. 10(2004)1039 “Sulfated Polymers in biological mineralization: a plausible source for bio-inspired engineering”
[2] L. Addadi et al. Proc. Natl Acad. Sci USA 84 (1987) 2732-2736 “A Chemical Model for the Cooperation of Sulfates and Carboxylates in Calcite Crystal Nucleation: Relevance to Biomineralization”
[3] L. Ng et al. J. Struct. Biol. 143 (2003) 242-257 “Individual cartilage aggrecan macromolecules and their constituent glycosaminoglycans visualized via atomic force microscopy”
[4] S.H.Behrens et al. J. of Chem. Phys. 115(2001) 14, 6716-6721 “The Charge of Glass and Silica Surfaces”
[5] W.F.Heinz et al Nanotechnology 17(1999)143-150 “Spatially resolved force spectroscopy of biological surfaces using the atomic force microscope”
[6] J. Aizenberg et al. J. Am. Chem. Soc. 121 (1999) 4500-4509 “Oriented Growth of Calcite Controlled by Self-Assembled Monolayer of funcionalized Alkanethiols Supported on Gold and Silver”
[7] H.H. Teng et al. Geochimica et Cosmochimica Acta 64(2000)13, 2255-2266 “Kinetics of calcite growth: Surface processes and relationships to macroscopic rate laws”